The Coin battery

This is a experiment in the category of Natural Science Projects — most of them combine some Physical Science principle with electronics.
These will show you what practical applications there are for electronics in all aspects of Science. Did you know that the world abounds in batteries?
Virtually all metals can act as electrodes, and almost anything but distilled water can be used as an electrolyte. This characteristic of our world is evident everywhere we look. Metals eaten away by rust and corrosion can be said to have sacraficed themselves as the electrode of nature's big battery.
A battery is formed whenever two dissimilar (not the same type) metals are in contact with a liquid which is capable of acting chemically on the two metals. The liquid may be acidic or alkali as long as it can react on the metal to extract electrons or atoms.
There is an optimum chemical for use with any two metals, but many other chemicals will work acceptably as an electrolyte as long as they can conduct electricity. The voltage produced between the dissimilar metals depends on the chemical properties of the metals.
Someone has tested all possible combinations of metals to determine the relative voltage between any two metals. The listing (in order of voltage amplitude and polarity relative to hydrogen as a OV reference) is called the "electromotive series of the metals".
Choosing metals in this listing which are farthest apart in the list will yield the highest voltages. Current delivering abilities are related to other chemical properties, so don't expect the highest voltages to also be able to supply the most current.
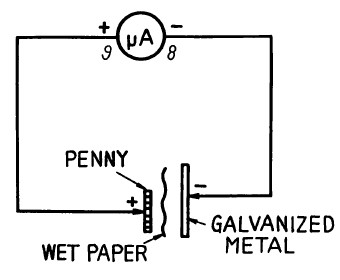
An abbreviated listing is included below. Notice that copper is widely separated from iron, zinc (used for galvanizing) and aluminum, which are all relatively active and therefore able to produce the required current for your "coin battery". A copper penny can be used as the (+) electrode and many of the other metals as (-) electrodes.

Place a piece of paper or cloth moistened with vinegar (as an electrolyte) between a penny and some other metal. Connect the Meter across this "battery" you have just made. The amount of Meter deflection is an indication of "battery" output. To compare the current delivering properties of different "coin batteries", use a 100 ohm Resistor in shunt (parallel) with the Meter. This increases the full-scale meter current from 0.25mA to about 2mA. It is an unusual penny battery that can pin this Meter circuit.
